Outlive: A Critical Review
Last updated 2025-07-04.
Outlive: The Science & Art of Longevity by Peter Attia (with Bill Gifford1) gives Attia’s prescription on how to live longer and stay healthy into old age. In this post, I critically review some of the book’s scientific claims that stood out to me.
This is not a comprehensive review. I didn’t review assertions that I was pretty sure were true (ex: VO2 max improves longevity), or that were hard for me to evaluate (ex: the mechanics of how LDL cholesterol functions in the body), or that I didn’t care about (ex: sleep deprivation impairs one’s ability to identify facial expressions).
First, some general notes:
- I have no expertise on any of the subjects in this post. I evaluated claims by doing shallow readings of relevant scientific literature, especially meta-analyses.
- There is a spectrum between two ways of being wrong: “pop science book pushes a flashy attention-grabbing thesis with little regard for truth” to “careful truth-seeking author isn’t infallible”. Outlive makes it 75% of the way to the latter.
- If I wrote a book that covered this many entirely different scientific fields, I would get a lot more things wrong than Outlive did. (I probably get a lot of things wrong in this post.)
- When making my assessments, I give numeric credences and also use terms such as “true” and “likely true”. The numbers give my all-things-considered subjective credences, and the qualitative terms give my interpretation of the strength of the empirical evidence. For example, if the scientific evidence suggests that a claim is 75% likely and I understand the evidence well, then I rate the claim as “likely true”. If I only read the abstract of a single meta-analysis, and the abstract unequivocally supports the claim but I’m only 75% sure that the meta-analysis can be trusted, then I rate it as “true”. Both claims receive a 75% credence.
Now let’s have a look at some claims from Outlive, broken down into four categories: disease, exercise, nutrition, and sleep.
Contents
- Contents
- Disease
- Exercise
- Nutrition
- Sleep
- Every animal sleeps
- We need to sleep 7.5 to 8.5 hours a night
- Basketball players who were told to sleep for 10 hours a night had better shooting accuracy
- Lack of sleep increases obesity and diabetes risk
- A study using Mendelian randomization found that sleeping <6 hours a night increased risk of a heart attack
- Lack of sleep causes Alzheimer’s disease
- Bonus
- Changelog
- Notes
Disease
People with metabolically healthy obesity do not have elevated mortality risk
A person is defined as having metabolic syndrome if they show at least three out of five symptoms:
- abdominal obesity (i.e. large waist circumference)
- high blood pressure
- high blood sugar
- high serum triglycerides
- low HDL cholesterol
People with obesity but no metabolic syndrome are said to have metabolically healthy obesity (MHO).
Here’s what Outlive has to say about MHO:
A large meta-analysis of studies with mean follow-up time of 11.5 years showed that people [with metabolic syndrome] have more than triple the risk of all-cause mortality and/or cardiovascular events than metabolically healthy normal-weight individuals. Meanwhile, the metabolically healthy but obese subjects in these studies were not at significantly increased risk. (page 95)2
My assessment:
- The statement “MHO subjects in these studies were not at significantly increased mortality risk” is technically correct: true (credence: 90%3).
- Obesity has no negative health effects for metabolically healthy people: false (credence: 5%).
- Metabolically healthy obesity does not increase mortality risk: highly unlikely (credence: 10%).
Outlive does not exactly say that MHO carries no elevated health risk, but some readers may come away with that impression, so I want to clarify that obesity is still bad for you even if you’re metabolically healthy.
The book cites Stefan et al. (2017)4 which in turn cites Kramer et al. (2013)5:
In a pooled analysis of 8 studies, metabolically healthy obese persons had a similar risk for all-cause mortality or CV [cardiovascular] events compared with the metabolically healthy normal-weight individuals (RR, 1.19; [95%] CI, 0.98 to 1.38). […] However, after we restricted analysis only to studies with at least 10 years of follow-up, the metabolically healthy obese group indeed had increased mortality and CV risk compared with the metabolically healthy normal-weight group (RR, 1.24; CI, 1.02 to 1.55; I^2 = 33.6%)
Stefat et al. (2017) also found that MHO subjects had higher rates of chronic disease than metabolically healthy normal-weight subjects, see Figure 2:
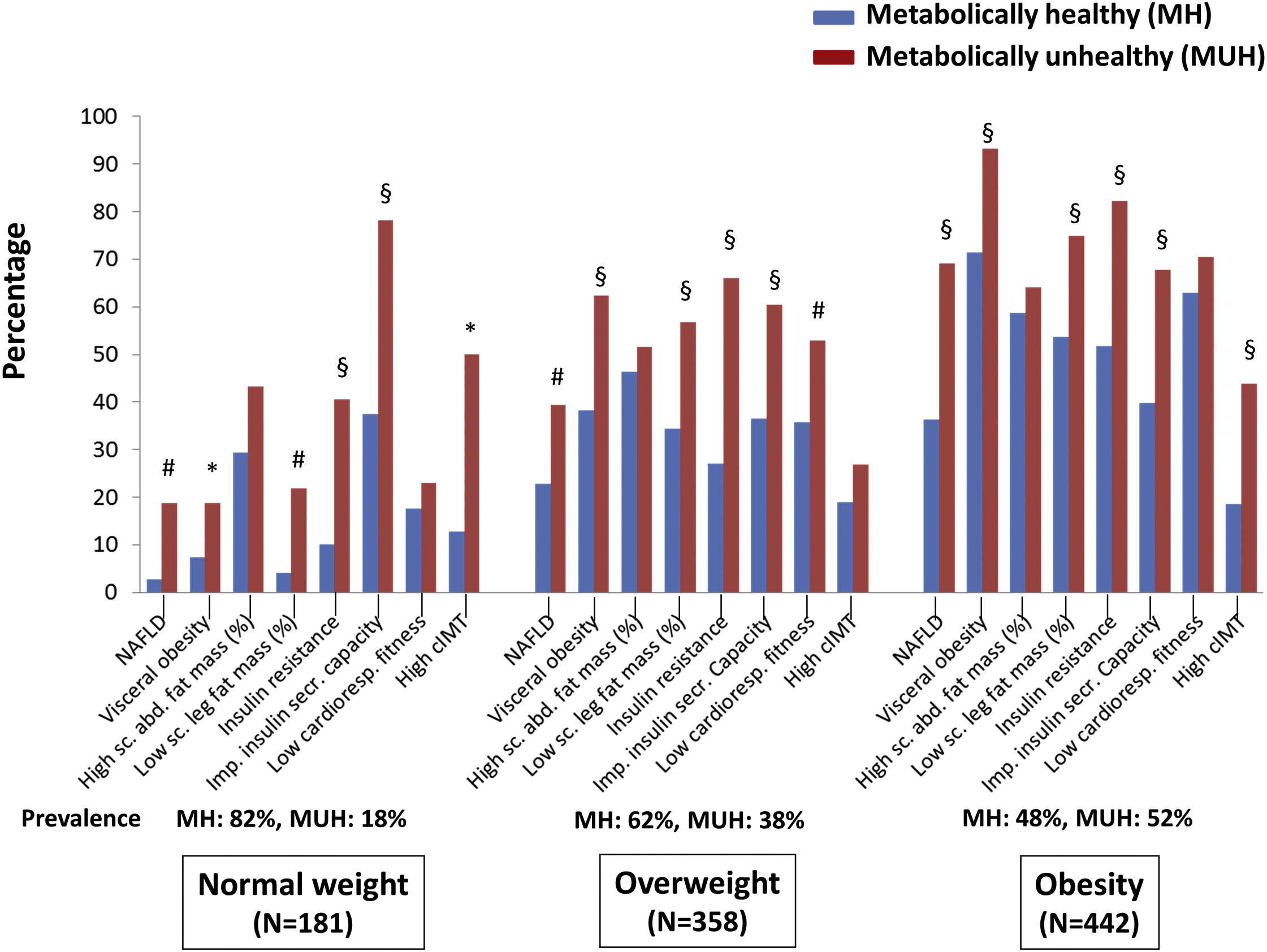
“MHO does not significantly increase risk” is one way you could describe this evidence. But it’s not the description I’d use:
- A relative risk (RR) of 1.19 sounds pretty bad. This finding is (just barely) not statistically significant, but it still has a likelihood ratio of about 5:1 compared to RR = 16—that is, we are 5x more likely to see this result if MHO elevates mortality risk by 1.19x than if it doesn’t elevate risk at all.
- MHO subjects had worse health across the board—higher rates of fatty liver disease, higher insulin resistance, lower cardiorespiratory fitness, more arterial plaque buildup—while also having non-significantly higher mortality. Which is a more reasonable interpretation: All those health problems somehow don’t translate into increased mortality? Or the health problems do increase mortality, and the observed RR = 1.19 is real even though it’s not statistically significant? (And the true RR probably isn’t exactly 1.19, but it’s somewhere in that vicinity.)
Many lament how often researchers treat p < 0.05 as “definitely real”, but it bugs me just as much when they treat p > 0.05 as “definitely no effect”.
Ben Carpenter at Red Pen Reviews writes:7
Roughly 50% of people8 with metabolically healthy obesity will develop at least one metabolic abnormality within 3–10 years, which is double the risk of normal-weight individuals. Further, metabolically healthy obesity is still associated with an increased risk of adverse cardiovascular events9, subclinical atherosclerosis10 (plaque buildup within the arterial walls), nonalcoholic fatty liver disease11, kidney function decline12, and type II diabetes13. So, considering this evidence, being metabolically healthy with obesity is probably short-lived and still has health risks.
Amyloid beta is implicated in Alzheimer’s disease
[Amyloid beta] is clearly bad stuff. (page 181)
My assessment: true (credence: 90%).
This claim stood out in my memory because a great deal of research on amyloid beta recently turned out to be fraudulent. But upon re-reading the relevant section of Outlive, I found that none of the book’s claims relied on the fraudulent research, and in fact the book cites the fraud investigation itself:
[Some scientists’] doubts seemed to be validated in July of 2022, when Science published an article calling into question a widely cited 2006 study that had given new impetus to the amyloid theory, at a time when it had already seemed to be weakening.14 The 2006 study had pinpointed a particular subtype of amyloid that it claimed directly caused neurodegeneration. That in turn inspired numerous investigations into the subtype. But according to the Science article, key images in that study had been falsified. (page 183–184)
The claims made in Outlive about the apparent negative effects of amyloid beta come from studies that predate the 2006 fraud, and those older claims appear to hold up. The book accurately describes the current state of the field as far as I understand it: amyloid beta is associated with at least some cases of Alzheimer’s, but research into amyloid beta has so far failed to uncover any useful treatments.
HDL cholesterol on its own doesn’t prevent heart disease
I wrote this claim in my notes on my first read-through, but upon re-reading, Outlive never actually said this. I’m including this claim anyway so that readers know I have poor reading comprehension and can’t be trusted.15
Here’s what the book actually said:
Risk does seem to decline as HDL-C rises to around the 80th percentile. But simply raising HDL cholesterol concentrations by brute force, which specialized drugs, has not been shown to reduce cardiovascular risk at all. (page 123)
My assessment:
- HDL cholesterol on its own doesn’t prevent heart disease: false, but Outlive never said that—in fact, it said the opposite.
Exercise
The book (rightly) focuses more on exercise than on nutrition or sleep. From what I can tell, it is the most scientifically accurate section of the book.
Update 2025-05-05: I’ve come to learn more about exercise and longevity, and I now believe some of Outlive’s claims on exercise are incorrect or overstated. The next three sections below explain my updated beliefs. They largely cover the same ground as my recent post, I was probably wrong about HIIT and VO2max, but with better evidence and citations (citing meta-analyses instead of tweets).
VO2max is the best predictor of longevity
VO2max is the maximum amount of oxygen your body is capable of consuming. VO2max is commonly used as a measure of aerobic fitness.
Outlive never directly says VO2max is the best predictor of longevity, but it sure implies it. The book spends eight pages talking about the association between VO2max and longevity, and about how important it is.
My assessment:
- Increasing VO2max increases longevity: true (credence: 97%).
- The causal relationship between VO2max and longevity is as strong as Outlive implies: false (credence: 10%).
- VO2max is the best proxy for physical fitness: false (credence: 20%) – performance on fitness tests (e.g. maximum pace on an incline treadmill) is easier to measure and probably more accurate.16
I have two objections to how Outlive characterizes VO2max:
- The book cites observational studies, not RCTs.
- VO2max is a proxy for physical fitness, not physical fitness itself. And there are better proxies.
Correlation is not causation. I am confident that training to increase VO2max does, in fact, increase longevity. But I believe observational studies overstate the magnitude of the effect.
I’ve looked at both observational studies and RCTs on the relationship between exercise and health, and my sense is that observational studies overstate the causal effect by about 2x. (It’s on my to-do list to write a post about why I believe this.) I don’t know about VO2max in particular, but I expect that only about half of its observed association with longevity is causal.
However, even after cutting the effect in half, exercise is still the best general-purpose17 intervention for longevity.
As for my second objection: VO2max is only a proxy for physical fitness. Here I will quote an article by elite running coach Steve Magness, because I can’t explain it any better than he did:
In practical terms, Vo2max is like knowing the size of a car’s engine, which is really important if we want to know about performance. But if we want to know whether that car has a chance to win the Daytona 500, engine size alone won’t tell us. We also need to to know about the size of the fuel tank, about its fuel economy, about how long its tires will hold up, and about all the other small components that translate the power of engine to the speed of the car. It’s the same in humans. Vo2max is one of many components that, taken together, tell us about our holistic aerobic or cardiorespiratory fitness.
[…]
Vo2max matters. But it’s just one component of many that make up both performance and aerobic fitness. And that’s important because if we return to the original claims that Vo2max is the key indicator of longevity, we’ll find that the majority of the studies cited did NOT even use Vo2max as the main variable. They used performance! In the majority of research, peak speed and incline during the [Vo2max] test was the main correlate to longevity.
The large study18 on 750,000 veterans that found a 4-fold higher mortality risk for low versus high fitness used peak speed and incline, not Vo2max. Same with the research19 on 120,000 individuals finding a 5x difference in the risk of early death.
[…] And as we can see in this meta-analysis20 looking at mortality and fitness, all but a handful of the included studies used an estimate based on speed or time.
You get the point.
And this is good news! It means you don’t need to go to a lab and measure your Vo2max. You don’t even need to worry about Vo2max itself (or your watche’s [sic] horrible estimation of it). All you need to do is focus on overall aerobic fitness. Which can easily be measured, compared, and improved in a number of ways that are less expensive and more accessible than Vo2max.
So, in short: most studies that measure “VO2max” are actually measuring performance on a fitness test. And this is good news, because it means if your performance is improving—if your mile time or your 5K time is getting faster—then you’re making real progress.
You should train VO2max by doing HIIT at the maximum sustainable pace
Outlive recommends doing high-intensity interval training (HIIT) to improve VO2max:
The tried-and-true formula for [VO2max training] is to go four minutes at the maximum pace you can sustain for this amount of time—not an all-out sprint, but still a very hard effort. Then ride or jog four minutes easy, which should be enough time for your heart rate to come back down to below about one hundred beats per minute. Repeat this four to six times and cool down. (page 249)
My assessment:
- The best exercise routine includes HIIT: likely true (credence: 85%).
- Longer intervals are better than shorter intervals: likely true (credence: 85%).
- HIIT should be done at the maximum sustainable pace: false (credence: 25%).
- HIIT is the best way to improve VO2max: likely true (credence: 70%).
Four-minute intervals are reasonable: a meta-analysis by Wen et al. (2019)21 found that ≥2-minute intervals produced bigger performance benefits than <2 minutes (although all interval durations improved VO2max).
But I have two concerns:
- Training at the maximum sustainable pace is a recipe for burnout. You should train at a pace that’s difficult, but not maximum effort.
- There is mixed evidence on whether HIIT is the best way to improve VO2max. Low-intensity training might be just as good.
For the first concern:
Taken literally, the quoted prescription is impossible to follow. If you do your first interval at the maximum sustainable pace, then your second interval cannot possibly be done at the same pace because you will be fatigued from the first interval.
Perhaps Attia meant to say that you should go at the maximum pace you can sustain across all intervals. That’s physically possible, but it still seems like a bad idea.
I wanted to cite RCT evidence comparing HIIT at different intensities while controlling for duration (e.g., 4-minute intervals at maximum pace vs. 4-minute intervals at 90% of maximum pace). But I couldn’t find any. Instead, the evidence on this question comes from studies of how athletes train.
Elite athletes typically do HIIT at roughly 90% of VO2max (Seiler (2010)22). This is considerably slower than the maximum sustainable pace (Billat (2001)23). For example, Billat et al. (1995)24 found that a sample of elite long-distance runners could maintain 90% of VO2max for an average of 16.55 minutes.25 If you can maintain a certain pace for 16 minutes straight, then you can certainly run at that pace for four 4-minute intervals with rests in between.
For the second concern: is HIIT really better than low-intensity training (LIT)26 for improving VO2max? Probably yes, but it’s unclear.
The RCT evidence on this is mixed. A meta-analysis by Wen et al. (2019)21 found that HIIT worked better than LIT for increasing VO2max. However, a review of meta-analyses by Crowley et al. (2022)27 found inconsistent evidence.28
I didn’t look at most of the meta-analyses reviewed by Crowley et al. I did look at Gist et al. (2013), which at a glance appears to be the most rigorous “contrarian” meta-analysis. It found that sprint interval training did not increase VO2max by more than endurance training. However, this is roughly consistent with Wen et al. (2019)21, which found that longer intervals (2+ minutes) worked better than sprint intervals.
RCTs may overstate the benefits of HIIT relative to LIT. A meta-analysis by Mølmen et al. (2024)29 found that HIIT rapidly improved participants’ fitness, whereas the benefits of LIT took longer to show up. Therefore, a 5-week study will show a clear advantage to HIIT even if LIT might be better in the long run:
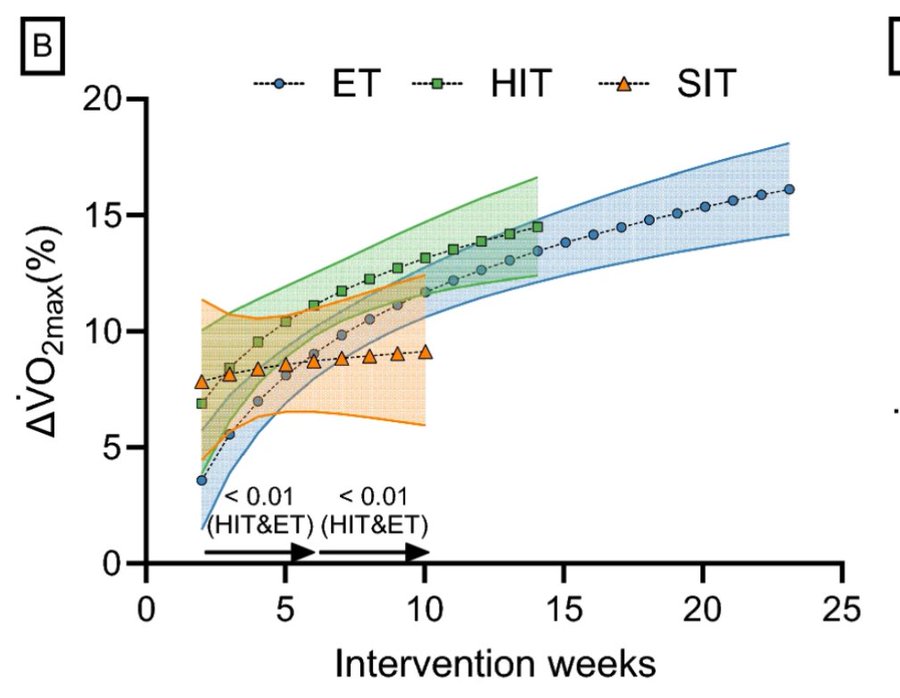
(ET = endurance training; HIT = high-intensity training; SIT = sprint-intensity training)
That being said, there is some evidence that HIIT provides benefits on top of LIT, so if your goal is to optimize longevity, then I believe it makes sense to do both.
You should do 3+ hours/week of zone 2 training and one or two sessions/week of HIIT
[I]t seems that about three hours per week of zone 2, or four 45-minute sessions, is the minimum required for most people to derive a benefit and make improvements, once you get over the initial hump of trying it for the first time. (page 243)
Even if we are not out to set world records, the way we train VO2max is pretty similar to the way elite athletes do it: by supplementing our zone 2 work with one or two VO2max workouts per week. (page 249)
My assessment:
- This is a good exercise routine: true (credence: 95%).
- This routine is uniquely better than any other: likely false (credence: 25%).
RCT evidence doesn’t tell us much about the optimal exercise routine. As with HIIT vs. LIT, the best evidence comes from looking at how top athletes train.
Elite athletes typically do 80% of their training at low/moderate intensity (a.k.a. zone 2), and 20% at high intensity (Seiler (2010)22; Stöggl & Sperlich (2015)30). For elite athletes, doing more than 20% of cardio sessions at high intensity can induce overtraining (Seiler (2010)22).
Outlive recommends training at just two intensities: low and high. But many elite athletes train at three or more different durations/intensities (Stöggl & Sperlich (2015)30). Different training modalities cause your body to adapt in different ways, so it makes sense to mix things up. You might do something like 80% zone 2 training, 15% long-duration (~4-minute) interval training, 5% sprint (< 1 minute) interval training. But just two intensities is probably sufficient to get the vast majority of longevity benefits.
I am not confident that ordinary folks should follow the same 80/20 rule as elite athletes. Professionals train 20–30 hours per week, including 4+ hours of high-intensity training. If I do 5 hours a week of cardio instead of 20, do I really need to restrict my HIIT to only one hour? Maybe I could handle 2 or 3 hours (or even 5 hours) without overtraining. But in the absence of direct evidence, I’ll follow the 80/20 rule.
For the record, my exercise routine is:
- Low-intensity cardio 3x/week for 45 to 90 minutes (depending on how I’m feeling);
- HIIT once every 1–2 weeks (usually four 4-minute intervals, and occasionally a different variation);
- Resistance training 3-4x/week.
I think this is less than the optimal amount of HIIT, but I don’t like doing HIIT.
Stability is as important as cardiovascular fitness and strength
Outlive claims that exercise is the best way to increase longevity, and that the ideal exercise programs includes four components:
- long-duration, low-intensity cardio (“zone 2” training)
- high-intensity interval training (VO2 max training)
- strength training
- stability training
An abundance of evidence shows that the first three types of exercise are excellent for health and longevity. The fourth is not so well-supported.
[Stability] is the foundation on which our twin pillars of cardiovascular fitness and strength must rest. (page 265)
My assessment: I don’t know how to evaluate this. It seems to border on not even wrong.
The book defines stability as “the subconscious ability to harness, decelerate, or stop force”, while admitting that this isn’t a great definition. I don’t know how to empirically test stability by this definition. You can formally define cardiovascular fitness as VO2 max (or resting heart rate, etc.), and then show that improving your chosen metric improves health and reduces mortality risk. But I don’t know how to operationalize “stability”.
(On one reading, this definition of stability sounds nearly identical to “strength”, because your ability to decelerate/stop force is pretty much entirely determined by your ability to generate force.)
The chapter on stability only made two-ish testable claims that I could identify. The first:
DNS [dynamic neuromuscular stabilization] originated with a group of Czech neurologists who were working with young children with cerebral palsy in a hospital in Prague in the 1960s. They noticed that because of their illness, these kids did not go through the normal infant stages of rolling, crawling, and so forth. Thus they had movement problems throughout their lives. But when the children with cerebral palsy were put through a “training” program consisting of a certain sequence of movements, replicating the usual stages of learning to crawl, sit up, and eventually stand, their symptoms improved and they were better able to control their motions as they matured. The researchers realized that as we grow up, most healthy humans actually go through an opposite process—we lose these natural, healthy, almost ingrained movements. (page 270)
(The book cites Frank et al. (2013)31.)
TLDR: Some research found that DNS helped children with cerebral palsy, so it might also help adults prevent injuries. The assertion that it would help adults is presented without evidence, and to my knowledge, no evidence exists.
Relatedly, Outlive cites an interview Peter Attia did with “leading American practitioner of DNS” Michael Rintala, D.C. (a.k.a. Doctor of Chiropractic). Chiropractic is pseudoscience and chiropractors are fake doctors, so without having looked into this much, I’m pretty skeptical of DNS. If Rintala practices bogus medicine in one arena, that’s evidence that his DNS research is also bogus.
I don’t know that this first claim is false, but it’s essentially pulled out of thin air so I have no reason to believe that it’s true.
Outlive makes a second semi-testable claim: it implies (but does not explicitly state) that squatting with perfect form has lower injury risk than squatting asymmetrically. This claim sounds intuitively plausible but I could not find any supporting evidence (and there’s some evidence that “bad” squat form isn’t necessarily bad32).
And squatting asymmetrically is probably better than not squatting at all (credence: 90%) because (1) studies find robust health benefits to strength training and (2) probably most of the subjects in those studies don’t have particularly good form.
Outlive didn’t do a good job of defining “stability training”, so I’ll do it myself. Let’s say the purpose of stability training is to reduce the risk of falling. In that case, is stability training useful?
- A meta-analysis by Sherrington et al. (2011)33 found that essentially any kind of exercise reduced fall risk, with balance training having a 22% larger effect than an “average” exercise program, and long-duration exercise (>50 hour trial duration) having a 23% larger effect than “average”. So “stability training” does appear to work, and high-dose exercise programs that included balance training reduced fall risk by 38%.
- A meta-analysis by de Souto Barreto et al. (2019)34 found that exercise significantly reduced falls (RR = 0.88) and injurious falls (RR = 0.74), and non-significantly reduced fractures (RR = 0.84, 95% CI 0.71–1.00). It compared exercise programs by type: (1) aerobic, (2) strength, (3) other (tai chi/dance), (4) multicomponent (aerobic + strength + balance). The meta-analysis did not find any significant differences between four types. (And it wasn’t a p = 0.06 situation either—most of the p-values were greater than 0.8.)
- There’s some contrary evidence on balance training. A meta-analysis by Kümmel et al. (2016)35 found that balance training on a particular task improves performance on that task, but does not transfer even to very similar tasks. This gives reason to doubt that balance training reduces injury risk.
So exercise appears to help with stability. And balance exercises might work better than other types of exercise, but they might not work at all.
My assessment of some claims that Outlive didn’t make, but sort of implied:
- Stability is useful: likely true (credence: 90%).
- Exercise can improve stability: likely true (credence: 80%).
- Exercising to improve stability matters as much as exercising to reduce cardiovascular disease/diabetes/cancer: almost certainly false (credence: 5%).
- Most people should do additional stability training on top of the cardio and strength training that they should already be doing: likely false (credence: 15%).
Nutrition
Rhesus monkey studies suggest that calorie restriction improves longevity but only if you eat a fairly unhealthy diet
I won’t provide a direct quote from Outlive because it would be too long. To summarize, the book says (pages 312–316):
- A 2009 University of Wisconsin-Madison (UW) study36 found that rhesus monkeys on a calorie-restricted diet lived longer than the control group.
- But a similar 2012 study37 by the National Institute of Aging (NIA) found that a calorie-restricted diet did not improve longevity.
- The biggest difference between the studies was that the UW monkeys ate processed food and the NIA monkeys ate a whole-foods diet formulated by a primate nutritionist.
- So it looks like calorie restriction improves longevity if you eat mostly processed food, and doesn’t matter much if you eat a healthy diet.
My assessment:
- Outlive accurately summarizes these two studies: true (credence: 90%).
- Calorie restriction (CR) improves longevity but only if you eat a fairly unhealthy diet: likely true (credence: 70%).
- The rhesus monkey studies support the above claim: unclear (credence: 50%).
- The rhesus monkey studies generalize well to humans: somewhat unlikely (credence: 35%).
I found the book’s interpretation to be reasonable and appropriately couched in uncertainty but I want to write about the studies because they were interesting.
A 2017 collaboration38 by the authors of the UW and NIA studies reviewed the differences in study designs and outcomes. They agreed with Attia’s interpretation that diet quality was the most likely explanation for the different results, and that the studies jointly suggest that calorie intake is an important predictor of longevity—the monkeys in the NIA control group ate as little as those in the UW calorie restriction (CR) group.
I read the collaboration and noticed some results that don’t add up:
- In the NIA study, the control group developed more chronic disease than the CR group (Figure 6), which seemingly contradicts the finding that calorie restriction didn’t help.
- NIA split monkeys into young and old cohorts based on the age of each monkey when the study started. Within the young cohort, the CR group had less chronic disease (Figure 6), but had worse mean and median longevity (Table 2). Why do these two measurements point in opposite directions?
- CR reduced lifespan in the young NIA cohort, and the magnitude of the effect was larger than in the UW study, but it wasn’t statistically significant39 so of course the authors ignored it. I’m not saying it’s a real effect, I’m just saying if you get a result (even a non-significant result) that goes in the opposite direction of what you predicted, then you should take that as a cue that you’re missing something.40
- The NIA young male cohort saw a decrease in bodyweight and body fat while the other three NIA cohorts saw essentially no change. Presumably, the main mechanism of calorie restriction is that it prevents obesity, so we should see a longevity improvement among NIA young males and not among the other three cohorts. But that’s not what we see. Instead we see essentially no effect in old males/females and a negative effect in young males/females.
- In the NIA study, why did calorie restriction reduce average bodyweight for males but not for females (Figure 2)? The authors took this as evidence of “sexual dimorphism in the relationship between food intake and bodyweight”. That does not sound plausible to me.
- According to the authors: “In rodents, early onset CR is more effective in extending longevity than adult onset CR. For nonhuman primates it appears that CR, while beneficial when implemented in adulthood, does not improve survival when implemented in juveniles.” It’s suspicious that rodent studies and monkey studies produced opposite results in this respect.
The NIA control group monkeys ate about as much as the UW calorie-restricted monkeys, which the authors take as evidence in favor of the original hypothesis—lower caloric intake improves longevity. Maybe.41 Or maybe it was the higher-quality diet (irrespective of caloric intake), or some other difference between the two studies.42
If two studies conflict, I’m wary of making my hypothesis more complicated to fit the results. The hypothesis started as “CR improves longevity”, which the UW study supported. But when the NIA study produced contradictory evidence, the hypothesis became “CR improves longevity, unless you’re eating a healthy diet, in which case it doesn’t”.
The two studies together support this hypothesis, but they don’t distinguish it from other plausible hypotheses (see previous footnote, repeated here42).
And if you look closely at the NIA results, the hypothesis needs to become even more complicated: something like “CR improves longevity, but if you’re eating a healthy diet then it has no effect for old monkeys and a harmful effect for young monkeys, and the mechanism is presumably that monkeys with healthy diets tend to have lower rates of obesity, except that young males in the NIA study didn’t weigh much less than in UW so for young males the mechanism is some other thing, and also the young vs. old effect is reversed in rodents for some reason.”
The more complicated a hypothesis, the more supporting evidence it requires.
More broadly, I’m skeptical that CR studies on lab animals generalize to the real world. Peter Attia agrees:
CR’s usefulness remains doubtful outside the lab; very lean animals may be more susceptible to death from infection or cold temperatures. […] Furthermore, there is no evidence that extreme CR would truly maximize the longevity function in an organism as complex as we humans, who live in a more variable environment than the animals [studied]. While it seems likely that it would reduce the risk of succumbing to at least some [chronic diseases], it seems equally likely that the uptick in mortality due to infections, trauma, and frailty might offset these gains. (page 81)
Another fact that seems important to me, but that Attia and the UW/NIA authors didn’t discuss: the monkeys never exercised. They were permanently housed in small cages (Mattison et al. (2005)43). Some of the the UW monkeys did participate in a different study44 measuring their physical activity which moved them to a metabolic chamber, but the metabolic chamber was about the same size as the cages—i.e., too small for meaningful exercise.
Physical activity appears to largely or fully cancel out the harms of higher-calorie diets.45 Even if calorie restriction works for sedentary people, it’s less likely to improve health for folks who exercise regularly.
Returning to the original hypothesis—”CR improves longevity but only if you eat a fairly unhealthy diet”—these studies provide a small amount of evidence for the hypothesis but not much. That said, the hypothesis sounds correct to me:
- People with unhealthy diets tend to overeat, so eating less would probably improve their health.
- People who get most of their calories from healthy sources (whole grains, nuts, etc.) are much less likely to overeat, so there’s no point in calorie restriction.
The data are unclear on whether reducing saturated fat intake is beneficial
(Note: SFA = saturated fatty acid, MUFA = monounsaturated fatty acid, PUFA = polyunsaturated fatty acid)
A more recent publication by the Cochrane Collaboration, published in 2020 as a 287-page treatise titled Reduction in Saturated Fat Intake for Cardiovascular Disease46, looked at fifteen RCTs in over fifty-six thousand patients and found, among other things, that “reducing dietary saturated fat reduced the risk of combined cardiovascular events by 17%.” Interesting. But the same review also found “little or no effect of reducing saturated fat on all-cause mortality or cardiovascular mortality.”
[…]
The data are very unclear on this question, at least at the population level. […] [A]ny hope of using broad insights from evidence-based medicine is bound to fail when it comes to nutrition, because such population-level data cannot provide much value at the individual level when the effect sizes are so small, as they clearly are here. (pages 337–338)
And a bonus quote:
If, after reading this chapter, you’re upset because you don’t quite agree with some detail I’ve covered—be it the ratio of MUFA to PUFA to SFA, or the exact bioavailability of soy protein, the role of seed oils and lectins, or the ideal target for average blood glucose levels […], I have one final piece of advice. Stop overthinking nutrition so much. Put the book down. Go outside and exercise. (page 346)
Well joke’s on you, I already exercised today, and now I’m back to over-analyze saturated fat.
My assessment:
- Saturated fat is unhealthy in expectation: likely true (credence: 85%).
- It’s a good idea for most people to reduce their SFA intake: possible (credence: 50%).47
- It’s a good idea for people with high cholesterol to reduce their SFA intake: likely true (credence: 70%).
- The data are unclear: unclear. (Yes, it’s unclear whether the data are unclear. It depends on how much clarity you want.)
- The amount of SFA in your diet doesn’t matter all that much: possible (credence: 50%).
Attia’s position on saturated fat stuck out to me because the mainstream view says saturated fat is unhealthy. After spending much longer on this than I’d originally planned, I’ve come to the conclusion that the mainstream advice is basically reasonable, and Attia’s position is also basically reasonable. There’s some evidence that reducing SFA is beneficial and there’s little evidence to the contrary, but (a) the evidence is only moderately strong at best, and (b) there’s a lot of variation in how SFA affects people, so you might not need to worry about it unless you have high cholesterol.
Observational studies have found mixed results, with the more reliable studies generally finding moderate associations between saturated fat and heart disease. For example, Zong et al. (2016)48 found that shifting 1% of daily calories from saturated fat to polyunsaturated fat was associated with an 8% reduction in coronary heart disease (p < 0.001). But observational studies don’t establish causality, so let’s look at randomized controlled trials (RCTs).
A 2020 Cochrane review46 of RCTs found that replacing dietary saturated fat significantly reduced cardiovascular events and LDL cholesterol levels, and non-significantly reduced all-cause mortality and cardiovascular mortality. (Some other meta-analyses of RCTs have been done,49 but the Cochrane review likely has the strongest methodology.)
It’s not entirely clear how to interpret the results of the Cochrane review. Out of eight primary outcome variables (including all-cause mortality, cardiovascular mortality, etc.), reducing SFA only statistically significantly improved one variable. But it showed a positive effect for all eight. This weakly suggests that there’s a real effect and the RCTs were underpowered for most of the measures. If we treated the eight measures as independent, this would constitute strong evidence of a real effect, but the measures are mostly correlated with each other.
The Cochrane review looked at dozens of other outcome variables. Most importantly, replacing saturated fat significantly reduced LDL cholesterol. (A WHO (2016)50 meta-analysis of 84 RCTs agreed with this result.) Cholesterol-lowering drugs have been shown to lower all-cause mortality (see Navarese et al. (2018)51 and Ennezat et al. (2022)52), so it stands to reason—although we only have weak direct evidence—that if reducing SFA improves LDL cholesterol, then it should improve all-cause mortality. Somewhat contradicting this, an RCT by Bergeron et al. (2019)53 found that SFA caused the body to produce mainly larger LDL particles, which are less harmful than small particles.
Sacks et al. (2017)54 performed a review with stricter inclusion criteria, looking only at RCTs that:
- controlled subjects’ dietary intake;
- lasted at least 2 years;
- proved adherence by measuring biomarkers like cholesterol;
- did not replace saturated fats with trans fats in the intervention group.
Only four trials met these criteria. Three of the four were included in the Cochrane review; the fourth, the Finnish mental hospital study55, was excluded for using a cluster-randomized design instead of full randomization. All four of these studies supported the hypothesis that reducing SFA improves cardiovascular health, and they had a weighted average relative risk of 0.71 (95% CI 0.62–0.81, see Figure 2).
Two of the studies in the Cochrane review showed an increase in cardiovascular events when reducing SFA:
- The Sydney Diet Heart study56 had some study participants replace saturated fat with trans-fat-heavy margarine, which likely explains the increase in bad outcomes.
- The Rose study57 did not have any glaring problems like Sydney Diet Heart, but it lasted for less than 2 years and only had a total of 54 participants. I take it as valid but weak contradictory evidence.
It looks reasonably likely, but not conclusive, that saturated fat is unhealthy. How unhealthy?
The Cochrane review suggests that an intervention to reduce dietary SFA should prevent one cardiovascular event per 290 person-years and one death per 2300 person-years for the sort of people who participated in these trials (i.e., people with elevated baseline risk of cardiovascular disease).58 Compare to exercise, which is associated with a reduction of about 1 death per 300 person-years in individuals with chronic diseases.59
It’s not clear how to estimate the improvement in mortality for the general population. Participants in these trials died at approximately the normal rate (compare to US CDC mortality statistics) which suggests the effect should be similar, but it makes sense in theory that dietary interventions should have larger effects on unhealthy populations.
People should take omega-3 supplements
There is some evidence that supplementation with the omega-3 fatty acid DHA, found in fish oil, may help maintain brain health[.] (page 200)
[U]nless they are eating a lot of fatty fish, filling their coffers with marine omega-3 [fatty acids], [my patients] almost always need to take EPA and DHA supplements in capsule or oil form. (page 339)
My assessment:
- Omega-3 fatty acids improve brain health: likely true (credence: 75%).
- Omega-3s improve health in general: likely true (credence: 80%).
- It’s a good idea for most people to take omega-3 supplements: somewhat likely (credence: 65%).
A meta-analysis of RCTs by Dighriri et al. (2022)60 found that “[c]onsumption of omega-3 improved learning, memory ability, cognitive well-being, and blood flow in the brain.”
A Cochrane review61 of RCTs found no statistically significant effect of omega-3 consumption on all-cause mortality, cardiovascular events, stroke, or arrhythmia, and a weakly statistically significant effect on cardiovascular mortality, coronary heart disease mortality, and coronary heart disease events. The non-significant effects were all positive, except for a small negative effect on stroke.62
So omega-3s probably improve brain health, and they might have a small effect on heart health but it’s unclear.
As far as we can tell, there are no significant downsides to dietary omega-3s, so they easily pass a cost-benefit analysis as long as you don’t mind eating omega-3-rich foods.
The cost-benefit analysis for omega-3 supplementation is a bit murkier because supplements sometimes contain contaminants.6364 Raab et al. (2016)65 tested 67 supplements and found that all had safe levels of heavy metals, but did not test for mercury. Winwood (2013)66 claims that algae oil typically comes from algae grown in tanks and thus can’t be contaminated by heavy metals in the ocean.
I personally take a daily algae oil supplement. I take algae oil instead of fish oil because I’m vegan, but the potentially reduced risk of contaminants is a nice bonus.
Sleep
I read this chapter more skeptically than the others because it quoted Matthew Walker near the beginning. This raised some alarm bells because Walker wrote a bad book about sleep and has been caught manipulating data.67 Outlive cited a few of Walker’s papers to support certain claims, but none of those claims seemed particularly important so I didn’t review them.68
Every animal sleeps
Every animal engages in some form of sleep; scientists have found no exceptions, so far. (page 353)
My assessment:
- Every animal sleeps: somewhat unlikely (credence: 35%).
- It’s reasonable to assert that every animal sleeps: false (credence: 20%).
In support of this claim, Outlive cites Cirelli & Tononi (2008)69, which does not take a strong stance on whether all animals sleep:
Only a small number of species—mostly mammals and birds—have been evaluated in detail with respect to sleep. Most studies found signs of sleep, both behavioral (quiescence and hyporesponsivity) and electrophysiological (e.g., the slow waves of non-rapid eye movement (NREM) sleep). Scientists have been hesitant to attribute sleep to reptiles, amphibians, fish, and especially invertebrates, preferring the noncommittal term “rest” in the absence of electrophysiological signs resembling those of mammals and birds.
Cirelli & Tononi (2008) references some examples of animals that have been claimed not to sleep (particularly bullfrogs), but says the evidence is weak.
I don’t think Cirelli & Tononi supports Attia’s claim; it would be more accurate to say “no animal has been proven not to sleep”.
But other sources would disagree with this. For example:
It now appears that many species reduce sleep for long periods of time under normal conditions and that others do not sleep at all, in the way sleep is conventionally defined.
From Kushida, C. (2013). Encyclopedia of Sleep, Volume 1, page 38 (h/t Alexey Guzey).
Attia’s claim “scientists have found no exceptions” is sort of true in the sense that we haven’t found any definitive exceptions, but the claim “every animal engages in some form of sleep” isn’t well-established either.
We need to sleep 7.5 to 8.5 hours a night
[M]any, many studies have confirmed what your mother told you: We need to sleep about seven and a half to eight and a half hours a night. (page 354)
My assessment: false (credence: 10%).
(I could not find a citation for the quoted assertion.)
The most authoritative source on this question appears to be the National Sleep Foundation panel70, where sleep scientists were surveyed on their beliefs. The median panelist believed that 7 to 9 hours a night is “appropriate” for adults age 25–64, and 6 to 10 hours “may be appropriate for some people” in the same age range.71
The range given by Outlive (7.5 to 8.5 hours) is excessively narrow—according to sleep scientists, many people can/should sleep more or less than that.
My subjective uncertainty on this question mostly comes from the fact that I haven’t read any studies or even any meta-analyses and I’m only 90% confident that the National Sleep Foundation panelists know what they’re talking about.
Basketball players who were told to sleep for 10 hours a night had better shooting accuracy
In one study, Stanford basketball players were encouraged to strive for ten hours of sleep per day, with or without naps, and to abstain from alcohol or caffeine. After five weeks, their shooting accuracy had improved by 9 percent, and their sprint times had also gotten faster. (page 354)
I didn’t look into this study and I’m not giving a credence because I don’t really care about this particular claim. I bring it up because it contradicts the preceding claim that people should sleep 7.5 to 8.5 hours a night.
Lack of sleep increases obesity and diabetes risk
Even in the short term, sleep deprivation can cause profound insulin resistance. […] Multiple large meta-analyses of sleep studies have revealed a close relationship between sleep duration and risk of type 2 diabetes and the metabolic syndrome. (page 356)
My assessment:
- Sleep deprivation increases the risk of insulin resistance: highly likely (credence: 90%).
- Observational studies find relationships between short sleep duration and obesity/diabetes/metabolic syndrome: true (credence: 95%).
- Lack of sleep increases obesity and diabetes risk: highly likely (credence: 85%).
I only briefly investigated this but it passes a basic sanity check. I glanced at the papers cited by Outlive and they appear to support the quoted text. In addition, RCTs suggest that sleep restriction causes subjects to eat more and increases insulin resistance (a precursor to diabetes)—see meta-analysis by Reutrakul & Van Cauter (2018)72.
A study using Mendelian randomization found that sleeping <6 hours a night increased risk of a heart attack
[O]ne particularly interesting study compared observational and Mendelian randomization73 data in people with previous identified genetic variants that either increase or decrease their lifelong exposure to longer or shorter sleep duration. The MR data confirmed the observational findings, that sleeping less than six hours a night was associated with about a 20 percent higher risk of a heart attack. (page 359)
My assessment:
- A study found that sleeping <6 hours a night increases heart attack risk: true (credence: 98%).
- Sleeping <6 hours a night increases heart disease risk for most people: likely true (credence: 80%).
- This quote from the book does a good job of representing the state of the evidence: false (credence: 10%).
In support of this quote, Attia cites Dashti et al. (2019)74, which does not appear to say anything about heart attacks. I believe he meant to cite Daghlas et al. (2019)75 (on which Dashti is a co-author). Daghlas et al. (2019) supports Attia’s claim.
However, other Mendelian randomization studies have gotten different results. Zhuang et al. (2020)76 found no significant relationship between sleep duration and coronary heart disease; Yang et al. (2022)77 found a statistically significant but extremely weak (“probably not clinically relevant”) association78; while Liao et al. (2020)79 broadly agreed with Daghlas et al. (2019).
Out of four Mendelian randomization studies, two identified strong links between short sleep and heart disease/heart attack risk, and two suggested little to no effect. So while Outlive does accurately describe the results of a study, it misrepresents the evidence by ignoring the studies that contradict its thesis.
(I did not look into the quality of these studies, I just read their conclusions. It’s possible that the null-result studies are flawed in some way.)
The Mendelian randomization studies provide only weak to moderate evidence, but when combined with other evidence (such as the link to obesity discussed in the previous section), it appears reasonably likely that short sleep duration does indeed increase the risk of heart problems.
Lack of sleep causes Alzheimer’s disease
- Subsequent research … has pointed to chronic bad sleep as a powerful potential cause of Alzheimer’s disease and dementia. Sleep, it turns out, is as crucial to maintaining brain health as it is to brain function.
My assessment:
- Lack of sleep causes Alzheimer’s disease: possibly true (credence: 60%).
- The first sentence of the book quote is reasonable: true (credence: 90%).
- The second sentence of the book quote is reasonable: false (credence: 20%).
Some research has indeed found a link between bad sleep and Alzheimer’s, but it’s difficult to establish causality—see review article by Lloret et al. (2020)80.
The first quoted sentence from Outlive aligns with Lloret et al.’s summary of the literature. The second sentence converts a speculative hypothesis into a certainty.
Bonus
Dunning-Kruger effect
Looking back, I now realize that I was too far on the left of the Dunning-Kruger curve, caricatured below in figure 14—my maximal confidence and relatively minimal knowledge having propelled me quite close to the summit of “Mount Stupid.” (page 293)
The book’s Figure 14 reproduces this image from Wikimedia Commons:
My assessment:
- This graph accurately represents the Dunning-Kruger effect: false (credence: <1%).
- The existence of a “Mount Stupid” is supported by the scientific evidence: false (credence: 2%).
I’m willing to forgive this mistake because it doesn’t have anything to do with longevity, but it still bugs me.
If you search “Dunning Kruger” on Google Images, you will see a bunch of graphs that look like that, but no study on the Dunning-Kruger effect has ever produced empirical data with that shape.
Empirical results actually look something like this:
(Presumably, Attia found Figure 14 from the Wikipedia page on the Dunning-Kruger effect. I can’t really blame him for getting something wrong if he just pulled it from Wikipedia. And to Wikipedia’s credit, it has removed the incorrect image and replaced it with the correct one above.)
Changelog
- 2024-10-23: I added a reference to a meta-analysis of RCTs on balance training specificity. This provides stronger evidence for my previously weakly-held position that balance training isn’t useful. I updated my credences accordingly.
- 2025-05-05: I added three new sections on exercise:
- 2025-07-03: Previously I was inconsistent with how I reported credences for statements I thought were probably false—sometimes I reported my probability that the statement is true, and sometimes my probability that it’s false. I rewrote them so that all credences are given in terms of my probability that the statement is true.
- 2025-07-04: Made some minor changes in response to feedback.
Notes
-
The book is co-authored by Bill Gifford. It’s written from Attia’s point of view and some materials (such as Peter Attia’s website) approximately treat Attia as the sole author, so in my review I will credit the book’s claims to Attia and not to Gifford. ↩
-
Page numbers are from the 2023 Kindle edition of Outlive, ISBN 9780593236598, ebook ISBN 9780593236604. ↩
-
The meta-analysis did definitely find this result, but there’s some wiggle room around what “technically correct” means (because the meta-analysis found different results for different subgroups—I will discuss this shortly). So I’m only 90% confident. ↩
-
Stefan, N., Schick, F., & Häring, H. U. (2017). Causes, Characteristics, and Consequences of Metabolically Unhealthy Normal Weight in Humans. ↩
-
Kramer, C. K., Zinman, B., and Retnakaran, R (2013). Are metabolically healthy overweight and obesity benign conditions? A systematic review and meta-analysis. ↩
-
The standard method for significance-testing a relative risk is to assume that its logarithm follows a normal distribution. I did that and got an odds ratio of 7.27. But the sample mean of 1.19 is pretty far from the geometric mean of the 95% CI (1.16), and much closer to its arithmetic mean (1.18), so I redid the test with the assumption that the RR follows a normal distribution. The second method produces an odds ratio of 5.66, which I rounded down to 5 to be conservative.
It’s bad practice to run two different significance tests, but I think it’s okay in this case because I preferred the test that weakened my argument rather than strengthening it. ↩
-
The original article used PubMed links, which I replaced with DOI links and added full citations in footnotes. The quote is otherwise unchanged. ↩
-
Lin, H., Zhang, L., Zheng, R., & Zheng, Y. (2017). The prevalence, metabolic risk and effects of lifestyle intervention for metabolically healthy obesity. ↩
-
Eckel, N., Meidtner, K., Kalle-Uhlmann, T., Stefan, N., and Schulze, M. B (2016). Metabolically healthy obesity and cardiovascular events: a systematic review and meta-analysis. ↩
-
Kim, T. J., Shin, H. Y., Chang, Y., Kang, M., Jee, J., Choi, Y. H., Ahn, H. S., Ahn, S. H., Son, H. J., & Ryu, S. (2017). Metabolically healthy obesity and the risk for subclinical atherosclerosis. ↩
-
Chang, Y., Jung, H. S., Cho, J., Zhang, Y., Yun, K. E., Lazo, M., Pastor-Barriuso, R., Ahn, J., Kim, C. W., Rampal, S., Cainzos-Achirica, M., Zhao, D., Chung, E. C., Shin, H., Guallar, E., & Ryu, S. (2016). Metabolically Healthy Obesity and the Development of Nonalcoholic Fatty Liver Disease. ↩
-
Chang, A. R., Surapaneni, A., Kirchner, H. L., Young, A., Kramer, H. J., Carey, D. J., Appel, L. J., & Grams, M. E. (2018). Metabolically Healthy Obesity and Risk of Kidney Function Decline. ↩
-
Bell, J. A., Kivimaki, M., & Hamer, M. (2014). Metabolically healthy obesity and risk of incident type 2 diabetes: a meta‐analysis of prospective cohort studies. ↩
-
When Attia says the amyloid theory was weakening, he’s referring to the once-popular hypothesis that amyloid-beta is the sole cause of Alzheimer’s. That hypothesis now appears to be false, but amyloid-beta still looks relevant to Alzheimer’s somehow (it’s not quite clear how). ↩
-
Actually, I have very good reading comprehension in a relative sense—in the 98th or 99th percentile according to standardized tests. But 98th percentile reading comprehension still isn’t good enough to consistently understand the things you read, apparently. ↩
-
I’m not confident in the claim that they’re more accurate. I am not aware of any research directly comparing the predictive power of VO2max itself vs. performance tests. In theory, I would expect performance tests to be better predictors because they’re directly measuring your body’s physical capabilities. ↩
-
Some special-purpose interventions might work better. For example, if you’re a heavy smoker, quitting smoking might have a bigger effect than starting exercise. ↩
-
Kokkinos, P., Faselis, C., Samuel, I. B. H., Pittaras, A., Doumas, M., Murphy, R., Heimall, M. S. et al. (2022). Cardiorespiratory Fitness and Mortality Risk Across the Spectra of Age, Race, and Sex. ↩
-
Mandsager, K., Harb, S., Cremer, P., Phelan, D., Nissen, S. E., & Jaber, W. (2018). Association of Cardiorespiratory Fitness With Long-term Mortality Among Adults Undergoing Exercise Treadmill Testing. ↩
-
Harber, M. P., Kaminsky, L. A., Arena, R., Blair, S. N., Franklin, B. A., Myers, J., & Ross, R. (2017). Impact of Cardiorespiratory Fitness on All-Cause and Disease-Specific Mortality: Advances Since 2009. ↩
-
Wen, D., Utesch, T., Wu, J., Robertson, S., Liu, J., Hu, G., & Chen, H. (2019). Effects of different protocols of high intensity interval training for VO2max improvements in adults: A meta-analysis of randomised controlled trials. ↩ ↩2 ↩3
-
Seiler, S. (2010). What is Best Practice for Training Intensity and Duration Distribution in Endurance Athletes?. ↩ ↩2 ↩3
-
Billat, L. V. (2001). Interval Training for Performance: A Scientific and Empirical Practice. ↩
-
Billat, V., Renoux, J. C., Pinoteau, J., Petit, B., & Koralsztein, J. P. (1995). Times to exhaustion at 90,100 and 105% of velocity at V̇O2max (Maximal aerobic speed) and critical speed in elite longdistance runners. ↩
-
Blondel et al. (2001)81 found that a sample of physically active (but not elite) students could sustain 90% of VO2max for an average of 13.98 minutes. ↩
-
Different people use terminology in different ways. I am using LIT to refer to what Attia calls zone 2. Some studies call it moderate-intensity continuous training (MICT). Colloquially, it refers to an exercise intensity that you can sustain for a long time. Technically, it refers to exercise at or below the lactate threshold. ↩
-
Crowley, E., Powell, C., Carson, B. P., & W. Davies, R. (2022). The Effect of Exercise Training Intensity on VO2max in Healthy Adults: An Overview of Systematic Reviews and Meta-Analyses. ↩
-
It may be possible to resolve this inconsistency by digging deeper into the literature. Some relevant questions:
- Wen et al. (2019)21 found that long-duration HIIT worked better than short-duration. For the studies that find no benefit to HIIT over LIT, are they only looking at short-duration HIIT?
- Some RCTs match volume between groups. So if the HIIT group spends a total of (say) 16 minutes at high intensity, then the LIT group exercises for 16 minutes total. That’s not how people actually exercise. Do meta-analyses understate the benefits of LIT because they include volume-matched studies?
- How do the returns to HIIT vs. LIT differ for novice vs. experienced athletes?
- What happens when you combine HIIT with LIT?
-
Mølmen, K. S., Almquist, N. W., & Skattebo, Ø. (2024). Effects of Exercise Training on Mitochondrial and Capillary Growth in Human Skeletal Muscle: A Systematic Review and Meta-Regression. ↩
-
Stöggl, T. L., & Sperlich, B. (2015). The training intensity distribution among well-trained and elite endurance athletes. ↩ ↩2
-
Frank, C., Kobesova, A., and Kolar, P (2013). Dynamic neuromuscular stabilization & sports rehabilitation. ↩
-
I’m thinking in particular of Chiu (2023)82 which investigated knee valgus, a squat technique that was generally regarded as bad form, and found that it may be better than “correct” form. h/t Menno Henselmans. ↩
-
Sherrington, C., Tiedemann, A., Fairhall, N., Close, J. C. T., & Lord, S. R. (2011). Exercise to prevent falls in older adults: an updated meta-analysis and best practice recommendations.. doi: 10.1071/nb10056 ↩
-
de Souto Barreto, P., Rolland, Y., Vellas, B., & Maltais, M. (2019). Association of Long-term Exercise Training With Risk of Falls, Fractures, Hospitalizations, and Mortality in Older Adults. ↩
-
Kümmel, J., Kramer, A., Giboin, L. S., & Gruber, M. (2016). Specificity of Balance Training in Healthy Individuals: A Systematic Review and Meta-Analysis.. doi: 10.1007/s40279-016-0515-z ↩
-
Colman, R. J., Anderson, R. M., Johnson, S. C., Kastman, E. K., Kosmatka, K. J., Beasley, T. M., Allison, D. B., Cruzen, C., Simmons, H. A., Kemnitz, J. W., & Weindruch, R. (2009). Caloric Restriction Delays Disease Onset and Mortality in Rhesus Monkeys. ↩
-
Mattison, J. A., Roth, G. S., Beasley, T. M., Tilmont, E. M., Handy, A. M., Herbert, R. L., Longo, D. L., Allison, D. B., Young, J. E., Bryant, M., Barnard, D., Ward, W. F., Qi, W., Ingram, D. K., & de Cabo, R. (2012). Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. ↩
-
Mattison, J. A., Colman, R. J., Beasley, T. M., Allison, D. B., Kemnitz, J. W., Roth, G. S., Ingram, D. K., Weindruch, R., de Cabo, R., & Anderson, R. M. (2017). Caloric restriction improves health and survival of rhesus monkeys. ↩
-
Actually, the NIA young female cohort did see a statistically significant reduction in lifespan (p = 0.04), but it becomes non-significant if you do a Bonferroni correction. ↩
-
Mean and 95% confidence intervals for change in longevity from various study cohorts:
Cohort Mean 95% CI UW male 1.58 (-1.56, 4.72) UW female 2.22 (-2.74, 7.18) NIA young male -2.29 (-7.05, 2.47) NIA young female -4.79 (-8.88, -0.70) The CI for NIA young male contains the mean for UW male but the reverse is not true, and neither female cohort’s CI contains the mean of the other. ↩
-
If true, we would expect to find that calorie restriction worked for the NIA young male cohort because they ate about as much as their UW counterparts. But it didn’t work (in fact it shortened the average lifespan). ↩
-
I can think of several other hypotheses.
The UW control group ate ad libitum, which is fancy academic language for “as much as they want”. The NIA control group didn’t eat ad libitum. Instead, the researchers used previous data to determine how much monkeys tend to eat when fed ad libitum (controlling for age and bodyweight) and then fed subjects exactly that amount.
This brings to mind another hypothesis: Maybe some portion (let’s say 1/3) of monkeys tend to overeat, which causes health problems, and calorie restriction mainly only benefits the 1/3 who overeat. If the NIA control monkeys all received diets based on how much the average monkey eats, that prevents the most gluttonous 1/3 from overeating, so additional calorie restriction doesn’t produce meaningful benefits.
You could test this hypothesis using the UW data by dividing monkeys in the calorie restriction cohort into “high-calorie” and “low-calorie” group based on how much they ate ad libitum (controlling for age and bodyweight) and seeing if the high-calorie group had a bigger improvement in longevity than the low-calorie group. The groups would have small sample sizes so the result probably wouldn’t be statistically significant.
Some weak supporting evidence: in the UW study, the median longevity improvement was bigger than the mean improvement.
A fourth hypothesis: calorie restriction has a U-shaped effect on longevity, where a little calorie restriction helps, but excess calorie restriction increases mortality. (This is clearly true in the limit—100% calorie restriction certainly isn’t healthy.)
The studies weakly contradict this hypothesis. Figure 4 shows that NIA young males in the control group ate about as much as UW males, while in the other three control group pairings (NIA young female + UW female, NIA old male + UW male, NIA old female + UW female), the NIA group ate less. This predicts that calorie restriction should improve longevity in the NIA young male cohort but have a smaller or negative effect in the other three cohorts. But that’s not what the NIA study found. Instead, the young male and young female cohorts both saw decreased longevity from calorie restriction, and both old cohorts saw approximately no effect.
A fifth hypothesis: the studies have fundamental methodological issues that render the results invalid. The studies weakly support this hypothesis given how many peculiar and seemingly-contradictory findings I was able to identify.
I don’t know what those methodological issues might be. They could be things like:
- the different cohorts were managed by different researchers who used inconsistent procedures
- the cohorts had relevantly different genetic lineages
- the researchers fabricated data (probably not, but you never know)
- there was a mold infestation next to the control group’s cages
A sixth hypothesis: calorie restriction works, but only if you live in Wisconsin. ↩ ↩2
-
Mattison, J. A., Black, A., Huck, J., Moscrip, T., Handy, A., Tilmont, E., Roth, G. S., Lane, M. A., & Ingram, D. K. (2005). Age-related decline in caloric intake and motivation for food in rhesus monkeys. ↩
-
Yamada, Y., Colman, R. J., Kemnitz, J. W., Baum, S. T., Anderson, R. M., Weindruch, R., & Schoeller, D. A. (2013). Long-term calorie restriction decreases metabolic cost of movement and prevents decrease of physical activity during aging in rhesus monkeys. ↩
-
Ortega et al. (2016)83 summarizes the relevant literature. Several observational studies have found that overweight but physically fit individuals have little to no increase in mortality rates relative to normal-weight fit individuals (the largest study84 found a statistically significant but small effect (RR = 1.1); three other studies found no significant effect). By comparison, unfit people had 2–3x higher mortality than fit individuals. Figure 2 (reproduced below) summarizes the results from the four studies.
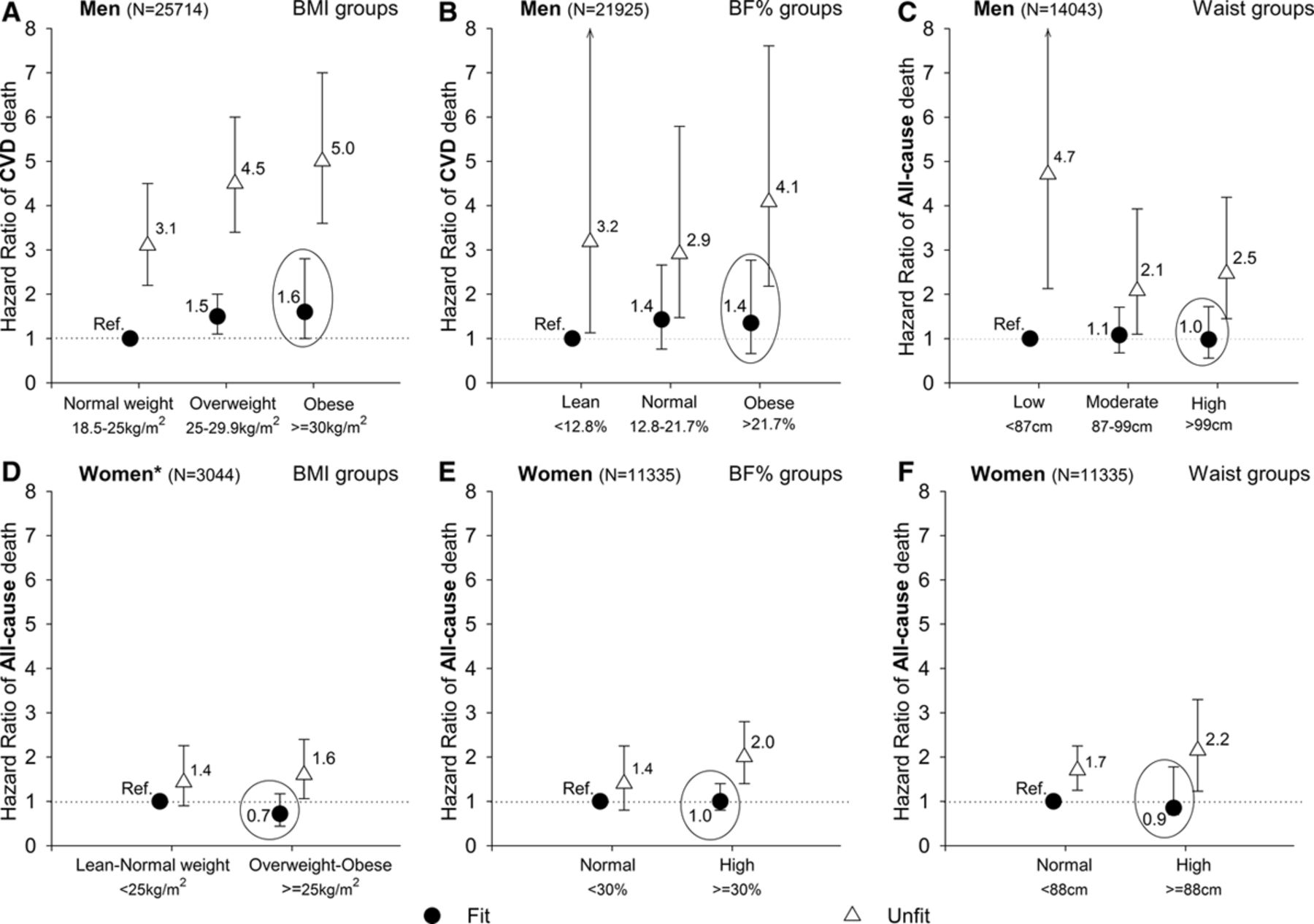
This finding from Ortega et al. (2016) is actually stronger than necessary for our purposes. It would be sufficient to say that exercise cancels out the harm of high-calorie diets by burning off the excess calories. But this shows that exercise (mostly) cancels out the harm even for people who don’t lose weight. ↩
-
Hooper, L., Martin, N., Jimoh, O. F., Kirk, C., Foster, E., & Abdelhamid, A. S. (2020). Reduction in saturated fat intake for cardiovascular disease. ↩ ↩2
-
Arguably, my credence for this latter claim should be higher than for the former claim, because reducing saturated fat has some chance of improving health and essentially no chance of harming health. But reducing saturated fat also has some costs (it makes your diet harder to follow).
In other words, on a cost-benefit analysis aimed at maximizing health, it’s clearly worth it to eat less saturated fat. But on an all-things-considered cost-benefit analysis, there’s more room for debate. ↩
-
Zong, G., Li, Y., Wanders, A. J., Alssema, M., Zock, P. L., Willett, W. C., Hu, F. B. et al. (2016). Intake of individual saturated fatty acids and risk of coronary heart disease in US men and women: two prospective longitudinal cohort studies. ↩
-
Heileson, J. L. (2019). Dietary saturated fat and heart disease: a narrative review. ↩
-
Mensink, R. P., & World Health Organization (2016). Effects of saturated fatty acids on serum lipids and lipoproteins: a systematic review and regression analysis. ↩
-
Navarese, E. P., Robinson, J. G., Kowalewski, M., Kolodziejczak, M., Andreotti, F., Bliden, K., Tantry, U. et al. (2018). Association Between Baseline LDL-C Level and Total and Cardiovascular Mortality After LDL-C Lowering. ↩
-
Ennezat, P. V., Guerbaai, R. A., Maréchaux, S., Le Jemtel, T. H., & François, P. (2022). Extent of LDL-cholesterol Reduction and All-cause and Cardiovascular Mortality Benefit: a Systematic Review and Meta-analysis. ↩
-
Bergeron, N., Chiu, S., Williams, P. T., M King, S., & Krauss, R. M. (2019). Effects of red meat, white meat, and nonmeat protein sources on atherogenic lipoprotein measures in the context of low compared with high saturated fat intake: a randomized controlled trial. ↩
-
Sacks, F. M., Lichtenstein, A. H., Wu, J. H. Y., Appel, L. J., Creager, M. A., Kris-Etherton, P. M., Miller, M. et al. (2017). Dietary Fats and Cardiovascular Disease: A Presidential Advisory From the American Heart Association.. doi: 10.1161/cir.0000000000000510 ↩
-
Miettinen, M., Karvonen, M., Turpeinen, O., Elosuo, R., & Paavilainen, E. (1972). EFFECT OF CHOLESTEROL-LOWERING DIET ON MORTALITY FROM CORONARY HEART-DISEASE AND OTHER CAUSES. ↩
-
Ramsden, C. E., Zamora, D., Leelarthaepin, B., Majchrzak-Hong, S. F., Faurot, K. R., Suchindran, C. M., Ringel, A. et al. (2013). Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. ↩
-
Rose, G. A., Thomson, W. B., & Williams, R. T. (1965). Corn Oil in Treatment of Ischaemic Heart Disease. ↩
-
The Cochrane review summary of findings reports that the interventions prevented 2 deaths per 1000 participants and the interventions lasted an average of 56 months, which equates to one death per 2300 pers ↩
-
See Arem et al. (2015)85 and Lee et al. (2022)86.
These were pooled analyses of cohort studies, not RCTs. I could not quickly find reliable RCT numbers.
Neither of these studies reported deaths prevented per person-year. I calculated the numbers using provided relative risks multiplied by number of deaths per person, divided by follow-up time. Number of years per death prevented varied based on exercise duration and intensity. I found that exercise prevented approximately one death per 300 years when defining “exercise” as 7.5+ MET-hours/week for Arem et al. (2015) and 150–224 minutes of moderate physical activity for Lee et al. (2022) (these two definitions are roughly equivalent).
There are a number of meta-analyses of RCTs, for example:
- Lawler et al. (2011)87 found exercise had a relative risk (RR) of 0.74 on all-cause mortality for individuals who had experienced heart attacks.
- Morishita et al. (2020)88 found RR = 0.76 on cancer mortality for cancer patients.
However, neither of these meta-analyses provided per-group mortality numbers or mean intervention length, so I can’t determine the number of years per death prevented without reading through every individual study. Based on the RRs, my guess is that these meta-analyses would give roughly similar numbers to the pooled analyses above.
To my knowledge, the most comprehensive meta-analysis is Posadzki et al. (2020)89, which reviewed 150 different Cochrane reviews and found an RR of 0.87 for all-cause mortality. But it provides even less information about the participants so I have no way of interpreting this number. ↩
-
Dighriri, I. M., Alsubaie, A. M., Hakami, F. M., Hamithi, D. M., Alshekh, M. M., Khobrani, F. A., Dalak, F. E. et al. (2022). Effects of Omega-3 Polyunsaturated Fatty Acids on Brain Functions: A Systematic Review. ↩
-
Abdelhamid, A. S., Brown, T. J., Brainard, J. S., Biswas, P., Thorpe, G. C., Moore, H. J., Deane, K. H. et al. (2020). Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. ↩
-
When talking about the calorie restriction studies, I said that a negative effect is a red flag even if it’s non-significant. In this case I’m not too concerned because:
- The effect on stroke was highly non-significant (p = 0.82). Compare to the NIA calorie restriction study which had p = 0.35 and p = 0.02 for young males and young females respectively.
- RCTs show omega-3s improve short-term brain function.
I think the most reasonable interpretation is that there’s no effect on stroke. ↩
-
Jacobs, M. N., Covaci, A., Gheorghe, A., & Schepens, P. (2004). Time Trend Investigation of PCBs, PBDEs, and Organochlorine Pesticides in Selected n−3 Polyunsaturated Fatty Acid Rich Dietary Fish Oil and Vegetable Oil Supplements; Nutritional Relevance for Human Essential n−3 Fatty Acid Requirements. ↩
-
Fernandes, A. R., Rose, M., White, S., Mortimer, D. N., & Gem, M. (2006). Dioxins and polychlorinated biphenyls (PCBs) in fish oil dietary supplements: Occurrence and human exposure in the UK. ↩
-
Raab, A., Stiboller, M., Gajdosechova, Z., Nelson, J., & Feldmann, J. (2016). Element content and daily intake from dietary supplements (nutraceuticals) based on algae, garlic, yeast fish and krill oils—Should consumers be worried?. ↩
-
Winwood, R. J. (2013). Algal oil as a source of omega-3 fatty acids. ↩
-
See also Walker’s response on why he presented the data the way he did. ↩
-
For example, the book cites Goldstein-Piekarski et al. (2015)90 (Walker being a co-author) for the passage “When we are deprived of REM, studies have found, we have a more difficult time reading others’ facial expressions.” I was skeptical of this statement even before knowing Walker co-authored the paper because it has the vibe of the sort of fun quirky result that doesn’t survive the replication crisis. But I don’t particularly care about this claim (it’s not actionable in any way) so I didn’t bother to investigate it.
(Also, this is a nitpick but the quoted passage says “studies have found” while only citing a single study.) ↩
-
Cirelli, C., & Tononi, G. (2008). Is Sleep Essential? ↩
-
Hirshkowitz, M., Whiton, K., Albert, S. M., Alessi, C., Bruni, O., DonCarlos, L., Hazen, N., Herman, J., Katz, E. S., Kheirandish-Gozal, L., Neubauer, D. N., O’Donnell, A. E., Ohayon, M., Peever, J., Rawding, R., Sachdeva, R. C., Setters, B., Vitiello, M. V., Ware, J. C., & Adams Hillard, P. J. (2015). National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. ↩
-
They gave different recommended sleep durations for different age ranges. This table reproduces all the recommendations for individuals age 6 and up (since I assume nobody under 6 is reading this):
Age Recommended May be appropriate 6–13 y 9 to 11 7 to 12 14–17 y 8 to 10 7 to 11 18–25 y 7 to 9 6 to 11 26–64 y 7 to 9 6 to 10 >64 y 7 to 8 5 to 9 -
Reutrakul, S., & Van Cauter, E. (2018). Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. ↩
-
Mendelian randomization is a technique for conducting a natural experiment. Instead of looking at heart attack risk as a function of sleep duration, you look at heart attack risk as a function of genes that determine sleep duration. The idea is that some confounding environmental variable might cause both shortened sleep and increased heart attack risk, but it can’t change subjects’ genes, so any observed relationship between genetic sleep duration and heart attack risk is probably causal.
I don’t have a strong opinion on how useful this technique is. ↩
-
Dashti, H. S., Jones, S. E., Wood, A. R., Lane, J. M., van Hees, V. T., Wang, H., Rhodes, J. A. et al. (2019). Genome-wide association study identifies genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. ↩
-
Daghlas, I., Dashti, H. S., Lane, J., Aragam, K. G., Rutter, M. K., Saxena, R., & Vetter, C. (2019). Sleep Duration and Myocardial Infarction. ↩
-
Zhuang, Z., Gao, M., Yang, R., Li, N., Liu, Z., Cao, W., & Huang, T. (2020). Association of physical activity, sedentary behaviours and sleep duration with cardiovascular diseases and lipid profiles: a Mendelian randomization analysis. ↩
-
Yang, Y., Fan, J., Shi, X., Wang, Y., Yang, C., Lian, J., Wang, N. et al. (2022). Causal associations between sleep traits and four cardiac diseases: a Mendelian randomization study. ↩
-
This study found about a 1% increased risk from short sleep, contrasted with Daghlas et al. (2019) which found about a 20% increased risk. ↩
-
Liao, L. z., Li, W. d., Liu, Y., Li, J. p., Zhuang, X. d., & Liao, X. x. (2020). Causal assessment of sleep on coronary heart disease. ↩
-
Lloret, M. A., Cervera-Ferri, A., Nepomuceno, M., Monllor, P., Esteve, D., & Lloret, A. (2020). Is Sleep Disruption a Cause or Consequence of Alzheimer’s Disease? Reviewing Its Possible Role as a Biomarker. ↩
-
Blondel, N., Berthoin, S., Billat, V., & Lensel, G. (2001). Relationship between run times to exhaustion at 90, 100, 120, and 140% of vV O2max and velocity expressed relatively to critical velocity and maximal velocity. ↩
-
Chiu, L. Z. (2023). “Knees Out” or “Knees In”? Volitional Lateral vs. Medial Hip Rotation During Barbell Squats. ↩
-
Ortega, F. B., Lavie, C. J., & Blair, S. N. (2016). Obesity and Cardiovascular Disease. ↩
-
Wei, M., Kampert, J. B., Barlow, C. E., Nichaman, M. Z., Gibbons, L. W., Paffenbarger Jr, R. S., & Blair, S. N (1999). Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. ↩
-
Arem, H., Moore, S. C., Patel, A., Hartge, P., Berrington de Gonzalez, A., Visvanathan, K., Campbell, P. T. et al. (2015). Leisure Time Physical Activity and Mortality. ↩
-
Lee, D. H., Rezende, L. F. M., Joh, H. K., Keum, N., Ferrari, G., Rey-Lopez, J. P., Rimm, E. B. et al. (2022). Long-Term Leisure-Time Physical Activity Intensity and All-Cause and Cause-Specific Mortality: A Prospective Cohort of US Adults. ↩
-
Lawler, P. R., Filion, K. B., & Eisenberg, M. J. (2011). Efficacy of exercise-based cardiac rehabilitation post–myocardial infarction: A systematic review and meta-analysis of randomized controlled trials. ↩
-
Morishita, S., Hamaue, Y., Fukushima, T., Tanaka, T., Fu, J. B., & Nakano, J. (2020). Effect of Exercise on Mortality and Recurrence in Patients With Cancer: A Systematic Review and Meta-Analysis. ↩
-
Posadzki, P., Pieper, D., Bajpai, R., Makaruk, H., Könsgen, N., Neuhaus, A. L., & Semwal, M. (2020). Exercise/physical activity and health outcomes: an overview of Cochrane systematic reviews. ↩
-
Goldstein-Piekarski, A. N., Greer, S. M., Saletin, J. M., & Walker, M. P. (2015). Sleep Deprivation Impairs the Human Central and Peripheral Nervous System Discrimination of Social Threat. ↩